Claremont removes former police officer accused of threats from city committees
CLAREMONT — In identical 5-3 votes Wednesday, the City Council removed Jonathan Stone from two committee appointments based on a recently released report alleging he threatened to murder and rape fellow officers and their family members while working...

Over Easy: ‘A breakfast without a newspaper is a horse without a saddle’
The Valley News was born in 1952 and so was I. This is probably coincidence, but I like to think there is more to our association than the fact that we are both from the year of the water dragon in the Chinese calendar.According to the Internet,...
Most Read
 Upper Valley winter shelters kept dozens warm and dry
Upper Valley winter shelters kept dozens warm and dry
 Former principal of South Royalton School released from prison
Former principal of South Royalton School released from prison
 Owner of Friesian horse facility ordered to pay care costs for seized animals
Owner of Friesian horse facility ordered to pay care costs for seized animals
Editors Picks
 Some families find freedom with Newport microschool
Some families find freedom with Newport microschool
 A Life: For Kevin Jones ‘everything was geared toward helping other people succeed’
A Life: For Kevin Jones ‘everything was geared toward helping other people succeed’
 Kenyon: Hanover stalls on police records request
Kenyon: Hanover stalls on police records request
 Editorial: Chris Sununu’s moral vacuum
Editorial: Chris Sununu’s moral vacuum
Sports

Woodstock boys lax defense steps up, holds off Hartford
WHITE RIVER JUNCTION — Monday night’s boys lacrosse game between host Hartford High and Woodstock came down to the final minute, with the Wasps prevailing, 11-10.The Vermont inter-division clash might have been decided, however, by a pair of goals at...
 Pick a sport and Pete DePalo’s has probably officiated it over the past 40-plus years
Pick a sport and Pete DePalo’s has probably officiated it over the past 40-plus years
 Lebanon girls lacrosse prevails over Coe-Brown
Lebanon girls lacrosse prevails over Coe-Brown
 Local roundup: Lebanon softball sweeps wins from Souhegan, Stark
Local roundup: Lebanon softball sweeps wins from Souhegan, Stark
 2024 Upper Valley high school tennis guide
2024 Upper Valley high school tennis guide
Opinion

Editorial: Gambling tarnishes America’s sporting life
Hey, Major League Baseball, does the name Pete Rose ring a bell? Remember him, “Charlie Hustle”? One of the game’s greatest players, whom you banned for life in 1989 because he bet on baseball games?We ask because you have on your hands another...
 By the Way: A white nationalist’s many mistruths
By the Way: A white nationalist’s many mistruths
 Column: The age-old question of what to read
Column: The age-old question of what to read
 Editorial: Transparency wins in NH Supreme Court ruling
Editorial: Transparency wins in NH Supreme Court ruling
 A Yankee Notebook: Among the crowds on vacation out West
A Yankee Notebook: Among the crowds on vacation out West

Photos

Clear and free in Hartford
 Ramping up their foraging
Ramping up their foraging
 Roadside assist in Bethel
Roadside assist in Bethel
 Preserving habitat in Etna
Preserving habitat in Etna
 Pitching in
Pitching in
Arts & Life

JAG Productions announces closure, citing ‘crisis facing the arts’
JAG Productions, the White River Junction theater company that has championed the work of Black, queer and trans artists, announced last week that it is closing in June after eight years of bringing groundbreaking work to the Upper Valley.Jarvis...
 How a hurricane and a cardinal launched a UVM professor on a new career path
How a hurricane and a cardinal launched a UVM professor on a new career path
 Out & About: Vermont Center for Ecostudies continues Backyard Tick Project
Out & About: Vermont Center for Ecostudies continues Backyard Tick Project
 Art Notes: After losing primary venues, JAG Productions persists
Art Notes: After losing primary venues, JAG Productions persists
 Over Easy: Marvels in the heavens, and in the yard
Over Easy: Marvels in the heavens, and in the yard
Obituaries
 Kenneth A. Curtis
Kenneth A. Curtis
West Lebanon, NH - Kenneth A. Curtis, age 64, passed Monday, April 15, 2024. A graveside service will be held 11am in the West Lebanon Cemetery on Thursday, May 2, 2024. Knight Funeral Home in White River Junction has been entrusted... remainder of obit for Kenneth A. Curtis
 Delores Howe
Delores Howe
Delores "Dee" Howe Hinesburg, VT - With the final lyrics of "King of the Road" wafting in the springtime air, our beloved Delores B. Howe crossed into the loving hands of our savior to reside in that spiritual mansion, that house not mad... remainder of obit for Delores Howe
 Jane Elizabeth Rooney
Jane Elizabeth Rooney
Manchester, NH - Jane Elizabeth Rooney, who had a long career as a social worker and psychotherapist, died on March 28, 2024, at Mount Carmel Rehabilitation and Nursing Center in Manchester, NH. Jane was born in Framingham, Massachuset... remainder of obit for Jane Elizabeth Rooney
 Cory John MacElman
Cory John MacElman
Lebanon, NH - Cory John MacElman, age 39, passed Friday, April 19, 2024. Ricker Funeral Home of Lebanon, NH is assisting the family. ... remainder of obit for Cory John MacElman


 Lawsuit accuses Norwich University, former president of creating hostile environment, sex-based discrimination
Lawsuit accuses Norwich University, former president of creating hostile environment, sex-based discrimination
 Local Roundup: Lebanon baseball, softball teams fall to Bow
Local Roundup: Lebanon baseball, softball teams fall to Bow
 In divided decision, Senate committee votes to recommend Zoie Saunders as education secretary
In divided decision, Senate committee votes to recommend Zoie Saunders as education secretary
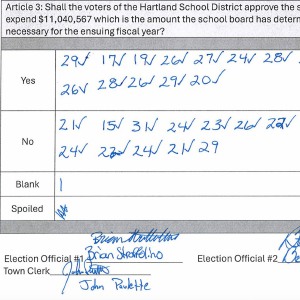 Hartland voters successfully petition for school budget revote
Hartland voters successfully petition for school budget revote